
HISTORY:
In 1906, Von Prowazek Haelberstaeder and discover inclusions in conjoncdvaux smear trachoma.
Moulder in 1964 shows that these microorganisms are intracellular bacteria development. They were designated in turn under the names: Bedsonia, Myagawanella,néorickettsies …
The order of chiamydiales (Greek = little jacket) with the only family Chiamydiaceae was created. The Chlomydiaceae were separated rickettsiae in 1970.
I – CLASSIFICATION:
The genus Chlamydia, within the family Chiamydiaceae, includes three species: Chlamydia trachomatis, Chlamydia psittaci and Chlamydia pneumoniae, previously designated as “TWAR strains” (Table I).
II – HOUSING AND TRANSMISSION:
1. Chlamydia psittaci infects birds and mammals. The bacteria is eliminated abundant in the feces of infected animals (birds, cats …) and is then present in the environment. Transmission is primarily by air by inhalation of contaminated dust.
2. Chlamydia pneumoniae infects only humans and human transmission is by air with disease outbreaks in communities.
3. Chlamydia trachomatis, has the exclusive host man. Serovars A, B, Ba and C responsible for trachoma is transmitted most often indirectly through contaminated hands, objects, flies. By officials against strains of urogenital infections or lymphogranuloma venereum are transmitted through sexual intercourse. Eye infections in adults caused by these strains are often associated with an inflammatory disease. The newborn is infected at the time of delivery.
III – PATHOPHYSIOLOGY:
The Chlamydia are obligate intracellular parasites that depend for their energy metabolism of the host cell. They bind to the cell surface specific receptors and then occurs endocytosis. Thus formed vacuole does not fuse with lysosomes. The multiplication of the bacterium will be inside the vacuole.
A to K serovars of Chlamydia trachomatis develop only in cylindrical epithelium: they are responsible for local infections. Serovars Ll, L2 and L3 invade lymphoid tissues and multiply in macrophages.
Chlamydia psittaci replicates in macrophages and is responsible for systemic infections.
Immune phenomena involved in Chlamydia infections are poorly known. Humoral immunity does not prevent recontamination and cellular immunity is unclear. While a first contact with the bacteria would give a mild impairment, repeated infections in the same patient would be responsible for severe symptoms observed (salpingitis example).
IV – PATHOGENICITY:
A – Chlamydia psittaci:
is responsible for the psittacosis. It is a lung infection of varying severity, sometimes complicated neurological manifestations (encephalitis).
B – Chlamydia pneumoniae:
causes broncho-pulmonary infections, generally mild, occurring especially in adolescents and young adults, but also in the elderly. These infections can be serious on ground debility.
C – Chlamydia trachomatis:
1. Trachoma:
It is endemic in inter-tropical areas. Linked to malnutrition, underdevelopment and lack of hygiene, it mainly affects children. Trachoma affects about 500 million people worldwide and is the leading cause of blindness. The disease is benign, but multiple infections favor maintenance of lesions and bacterial infections leading to the destruction of the cornea.
2. Sexually Transmitted Diseases:
Lymphogranuloma venereum or Nicolas Favre disease occurs mostly in developing countries. It begins with a small painless chancre, self-limited, genital or anal. Infection of satellite nodes will result in fistulizing adenitis may progress to chronicity.
Rectal injury can cause a narrowing of the rectum.
Other venereal infections with serovars D to K are common around the world, 75% of cases involve young adults 25 years of which at least 10% are infected. Transmitted through sex they translate:
– In humans by subacute urethritis occurring 10-60 days after a contaminant report. This urethritis is often asymptomatic. Chlamydia trachomatis is the main cause of acute epididymitis. Chronic prostatitis and of proctitis are also possible.
– In women, the infection often results in a discrete cervicitis. This can be complicated by salpingitis, peritonitis (pelvic inflammatory disease, peri-hepatitis Fitz-Hugh-Curtis syndrome). These high infections are responsible for sterility and ectopic pregnancies.
Chlamydia trachomatis has also been implicated in triggering Fiessinger Reiter syndromes (urethral damage, conjunctival and synovial) more common in males belonging to the HLA B 27 group.
– Infections of the newborn occurs during the transition from the infected birth canal. They result in conjunctivitis occurring in 5 to 15 days after delivery or a pneumonia observed after 1-3 months.
V – BACTERIOLOGICAL CHARACTERS:
A – Body type:
These are bacteria (containing DNA and RNA) immobile gram-negative.
They have an outer membrane containing lipopolysaccharide but not muramic acid. The bacteria in two forms:
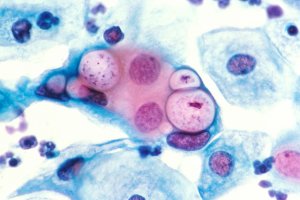
– The basic body, infectious particles, which have no metabolic activity. They are of small size (0.3 nm), spherical (Chlamydia trachomatis, Chlamydia psittaci) or pear shaped (Chlamydia pneumoniae) with a condensed nuclear device in periphery of the cytoplasm.
– The crosslinked body metabolically active, intracellular forms, which multiply by division. Of larger size (about 1 pm) they do not have a rigid membrane structure. The nuclear unit form in the cytoplasm loose frame. End of the cycle, they turn into intermediate body which then gives the elementary bodies. They accumulate in the center of the intracytoplasmic vacuole.
With Chlamydia trachomatis, a single vacuole is present in the cell, large, it pushes the nucleus at the periphery. It contains relatively few bacterial cells and contains glycogen. By cons with Chlamydia psittaci and Chlamydiapneumoniae, there may be several inclusions simultaneously. These are dense and do not deform the core.
When the cycle these inclusions rupture and release the elementary bodies that will in turn infect new cells. The in vitro multiplication cycle varies from 36 hours to 72 hours Chlamydia psittaci Chlamydia trachomatis and Chlamydiapneumoniae.
B – Antigenic Structure:
The antigenic structure is complex and Chlamydia antigens were gender specific, species and type (Table II).
1. The type of antigens:
There is a common lipopolysaccharide three species present in the outer wall and thermostable. It has structural similarities to that of Salmonella and antigenic cross-reactions with the rough shapes.
2. The species antigens:
The most important is the major outer membrane protein that plays a role porin; it can differentiate between them and species is used for the preparation of monoclonal antibodies.
3. The specific antigens of the types:
They can differentiate 15 serotypes of Chlamydia trachomatis and Chlamydia psittaci multiple serotypes. So far, only one serotype of Chlamydia pneumoniae is known.
VI – BIOLOGICAL DIAGNOSIS:
A – Samples:
The quality of the sample determines the search result of Chlamydia. It is important to bring the cells that contain the bacterial body which is formed by scraping the mucosa.
1. In case of lung infection:
Whatever the species Chlamydia in question, a simple nasopharyngeal swab enough. We may also use sputum, bronchial aspirates or bronchoalveolar washes.
2. In urogenital infections and eye:
One must make a smear of the mucous membrane. We can use a Dacron swab, cotton or plastic.
In humans, this deduction will be made in the urethra of 3-4 cm without drawing blood, the morning before urination.
In women, the collection will be made in the endocervix after cleaning to remove excess mucus. The sampling of the sensitivity can be increased by simultaneously making a smear urinary meatus.
During laparoscopy, it may be necessary to smear tubal or peritoneal cavity, or fluid collections present in the cul-de-sac.
For the diagnosis of chlamydial proctitis to one practice of rectal mucosa smear.
In conjunctivitis, samples will be taken in the conjunctival eyelid folds.
3. During lymphogranuloma venereum:
The infected node, if it is not fistulized will be punctured. Otherwise it will take a sample of pus.
4. Treatment of samples:
For direct research blade, it is necessary to smear, not too thick, then attach the blade to methanol.
For antigen research techniques by immunoassays, transport media for the sampling of conservation for 8 days at + 4 ° C are used.
For cultivation, we must remove the levy in the middle hypersaccharosé buffered (medium 2 SP). If the cultivation is done within 24 hours, the medium was stored at 4 ° C, if the crop is to be delayed, we must quickly freeze the sample at -80 ° C.
B – Direct diagnosis:
1. Research smear:
a) Staining:
Direct Search smear may occur after Lugol staining for research Chlamydia trachomatis: the inclusions appear purplish brown yellow brown background. This technique is inexpensive and insensitive reserved for trachoma screening. Giemsa stain is still very insensitive, and inclusions are difficult to identify: those of Chlamydia trachomatisappear clear with inside basophil granules; those of Chlamydia pneumoniae and Chlamydia psittaci are dark purple, very dense.
b) Direct immunofluorescence:
This is an excellent technique that highlights the bacterial body directly in smears. The use of monoclonal antibodies specific species allows for direct diagnosis of Chlamydia trachomatis and Chlamydia pneumoniae. The sensitivity and specificity of these techniques are excellent but they require an experienced observer and are difficult to use for large series of samples.
2. Search antigen in the sample:
These are mostly immune-enzyme techniques used. They involve polyclonal or monoclonal antibodies which have no species specificity. Only the genital and ocular specimens can be examined which limits their use in search of Chlamydia trachomatis. These techniques are sensitive but can provide false positive results. The use of confirmation reagents can limit these errors.
Automatable, these techniques are indicated for screening. There is also a technique using the chemiluminescence which is faster.
Methods of molecular biology (use of DNA probes or amplification chain polymérasee) could replace the previous methods in the near future.
3. Direct search by culture:
a) Culture embryonated egg:
Inoculation of embryonated egg is in the cavity yolk. This technique has the first Chlamydia isolates and was used for the preparation of antigens for serology. Currently it is abandoned in favor of the cell culture.
b) Cell cultures:
This is the reference method for the detection of Chlamydia, it can be used whatever the species and whatever the collection. However, it is difficult to implement and requires expensive equipment and trained personnel.
The most commonly used stem cells are the McCoy (semi-continuous lines of human origin) for Chlamydiatrachomatis and Chlamydia psittaci and HeLa (continuous line) for the 3 species.
Chlamydia psittaci and Chlamydia trachomatis strains Ll, L2 and L3 are highly virulent and the monolayer can be inoculated directly. By cons, with Chlamydia trachomatis and Chlamydia pneumoniae, must be centrifuged sampling with the cell culture to facilitate the adhesion of bacterial cells to cells.
The cells were then treated with cycloheximide to block protein synthesis of the host cell, respecting energy metabolism, which promotes the development of Chlamydia. Incubation was stopped after 2 or 3 days. The presence of Chlamydia is sought with an immunoenzymatic reaction with labeled antibodies or fluorescein.
NB Direct Search Chlamydia psittaci in samples must be done by taking great care to avoid accidental contamination of personnel.
C – Indirect diagnosis:
1. Complement fixation:
It uses the group antigen (LPS), thermostable. This technique is very sensitive but can be used in systemic infections (psittacosis, lymphogranuloma venereum) in some complicated forms of Chlamydia trachomatis infections (peritonitis), and in 25% of Chlamydia pneumoniae. This technique is faulted in most local infections, genital or lung.
Positive serology in complement fixation will also be positive (a title the same antibody) in micro-immunofluorescence.
2. Indirect micro-immunofluorescence:
Developed by Wang and Grayston, it is currently the gold standard for serology of Chlamydia trachomatis.
It allows the search for antibodies against three species of Chlamydia.
Although initially it was used for the typing of strains. For immunofluorescence, using 15 serovars of Chlamydiatrachomatis theoretically allows to define the type involved in the infection, but in practice a single strain is sufficient (eg L2 strain belonging to serovar D) due to multiple cross-reactions between different types. For this diagnosis is also used a strain of Chlamydia psittaci (Loth strain) and Chlamydia pneumoniae (strain TWAR or IOL).
The antigen can be either partially purified elementary bodies (microimmunofluorescence) or infected cells (fluorescent antibody inclusions).
The first method is the most common.
3. immunoenzymatic techniques:
They are also available, but for the moment there is no serum or reference antigen for serology and comparison with the microimmunofluorescence is difficult. As the latter is preferable.
4. Results of serology:
The micro-immunofluorescence is a sensitive method. However, its interpretation is difficult. Search IgM is disappointing for adults (it is against essential for the diagnosis of neonatal infection). The highlighting of IgA contributes little to the diagnosis of active infection. A title of high antibodies (> 1024) should suggest a complicated infection in women, even in the absence of overt clinical signs. Seroconversion is rarely observed, and a decrease of the title after antibiotic therapy. In other cases, serology provides little information in Chlamydia trachomatis. In fact, in patients titles change slowly and persist for years sometimes to high titers, even in the absence of reinfection .More anti Chlamydia trachomatis antibody are highlighted in 30 to 40% of patients. D is the same with Chlamydiapneumoniae.
The use of two (or three) different antigens may in some cases indicate which is the species responsible for the appearance of antibodies, but there are many cross-reactions between the three species of Chlamydia and a difference of securities with different antigens can not be observed at the beginning of an infection. So serology is of little value for the diagnosis of chlamydial infections. To try to improve his performance, techniques of “Westemblot” (or immunoréplique) are currently being studied.
VII – TREATMENT:
A – Preventive treatment:
In the absence of vaccine the fight against chlamydial infections through education, screening and the use of condoms for venereal diseases.
The fight against trachoma consists mainly of improved living conditions and hygiene.
B – Curative treatment:
The Chlamydia are sensitive to antibiotics that penetrate the cell. In uncomplicated infection must be used first and foremost tetracyclines, second generation (doxycycline, limécycline). In pregnant women, or in the new-born these products will be replaced by macrolides (erythromycin, roxithromycin …).
Fluoroquinolones may represent an alternative treatment of Chlamydia trachomatis.
Treatment should be sufficiently prolonged (15 days to 3 weeks). Before a venereal infection, it will look for partners and treat them.
In women, salpingitis, pelvic inflammatory disease, can lead to an associate used antibiotics (amoxicillin + clavulanic acid, metronidazole).


You must be logged in to post a comment.