Introduction:
Introduction:
Renal biopsy is an essential step in the study of kidney disease. It provides indispensable histological arguments which create the basis for the nosology of nephropathies, in particular glomeruli. Immunological marking and molecular biology studies have helped to clarify the clinical and histological individualization of kidney disease and have opened up many possibilities for their pathogenic understanding. The technical improvements made in recent decades have transformed the renal biopsy into a safe and informative technique, allowing it to play a central role in the nephrological diagnostic approach.
Renal biopsy sampling techniques:
Percutaneous renal biopsy:
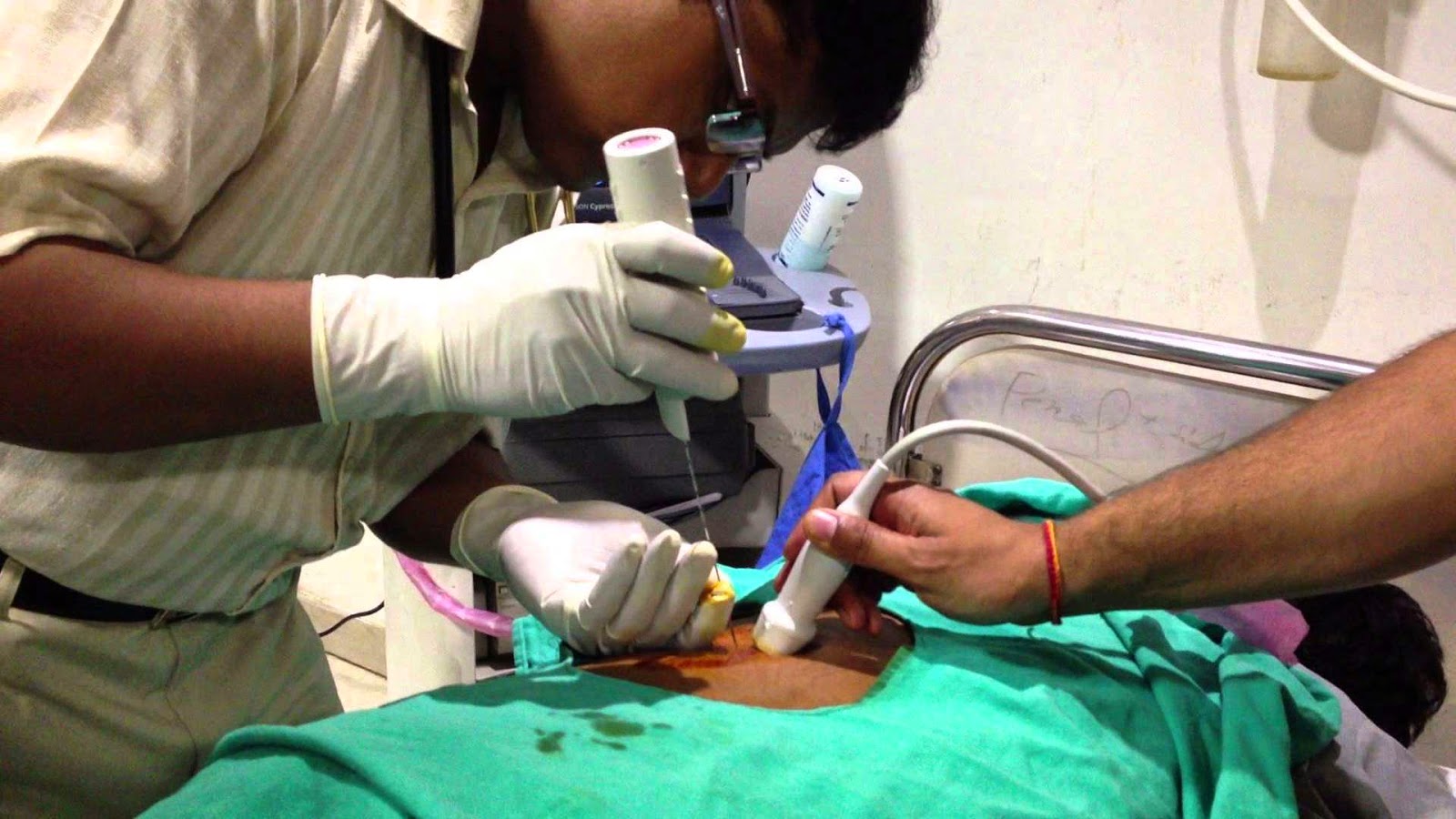
It is the most used and oldest renal biopsy method. It has the advantage of simplicity and reduced cost due to a short hospital stay of 24 to 48 hours. Since the 1950s, this technique has been constantly simplified and improved. Two major modifications have significantly increased the efficiency and especially the safety of the technique: the real-time guidance and the use of the automatic gun.
Recent studies report a success rate (diagnostic biopsies) of more than 99% and less than 0.1% of vital complications.Retrospective studies have shown a better efficacy of ultrasound-guided renal biopsies compared to techniques without direct visualization.
The use of the automatic gun also showed its superiority compared to the manual technique “on the needle”. The percutaneous renal biopsy under ultrasound control in real time with an automatic pistol has thus become the technique of reference.
Usually the patient is placed in a supine position on a hard plane and on a rolled pillow serving as a block compressing the abdomen and fixing the kidney. The puncture point is chosen preferably at the lower pole of the left kidney, less mobile than the right kidney, and with a lower risk of injury to the inferior vena cava. The sampling is carried out after local anesthesia with Xylocaine ® of the different cutaneomusculo-aponeurotic planes on the puncture path. The gun may be disposable or use disposable needles. The use of 14 gauge needles is preferable for clean kidneys. Their use is not accompanied by an increase in hemorrhagic complications compared to 16 gauge needles. The quality of the samples by the 18 gauge needles is insufficient with poor diagnostic yield.
The recommended duration of monitoring after biopsy of the native kidneys is 24 hours. The study by Whittier and Korbet shows that more than 90% of major complications are identified within the first 24 hours. The risk potentially incurred by the reduction of observation time does not seem justified. Surveillance includes clinical examination, assessment of pain, diuresis and hematuria, and regular measurement of blood pressure and heart rate. Resting in the supine position is recommended in the first 24 hours. Physical activity should be moderate for 8 to 15 days following the biopsy.
Transvenous renal biopsy:
Transvenous renal biopsy is an alternative renal biopsy technique developed over the last decade.
It is reserved for patients with contraindications to conventional percutaneous renal biopsy because the fragments taken are small, with a modified architecture.
The most frequent indication is that of uncorrectable coagulopathies or patients under anticoagulant treatment. She used the experience of endovascular liver biopsies. The transvenous biopsy technique uses the native vessels as a way of access to the renal parenchyma: internal jugular vein → vena cava → right renal vein. The theoretical advantages of this technique are:
• drainage of bleeding is mainly through the vein, thus limiting extravascular extravasation;
• the needle travels away from large vessels;
• lower risk of capsular perforation.
The main disadvantage is the small size of the predominantly medullary samples, and the difficulty of the techniques, which reduces the diagnostic yield of the technique. The feasibility of the technique has been demonstrated by Mal et al. in 1990.
Currently, two techniques are used: the transjugular PBR using a modified Colapinto aspiration system and, more recently, the “Quick-core” cutting system. The latter has a smaller diameter allowing deeper catheterization, without excessive fragmentation of the tissues. However, this system is accompanied by a higher incidence of capsular perforation, requiring systematic radiological monitoring during surgery to detect active bleeding and allow selective embolization. The modified Colapinto system has been used successfully by several groups in France and the Czech Republic. Cluzel et al. published the largest series of 400 transvaginal renal biopsies performed by this technique.They report a very small number of major complications (1%). In other series using the same aspiration technique, the rate of symptomatic complications is greater up to 18% of patients, most likely influenced by patient selection and the local policy of contraindication of percutaneous biopsies. The “Quick-core” technique appears to be accompanied by a higher rate of symptomatic complications (8%), and especially a large number of capsular perforations (74% of the biopsies). In this context, an additional precaution is required: selective embolization in case of extravasation of contrast medium after capsular perforation.
Surgical renal biopsy:
Two surgical techniques are described: “open-pit” renal biopsy and laparoscopy. These procedures are theoretically reserved for patients with percutaneous renal biopsy contraindications. Their use has become exceptional, taking into account the technical progress of the percutaneous biopsy and the success of the transvenous biopsy. The advantage of these techniques is mainly the visual control of hemostasis. They require general anesthesia, the risk of which must be taken into account when choosing the technique. This risk is assessed by the American Society of Anesthesiology (ASA) at three deaths per 1,000 in patients with moderate systemic disease, such as high blood pressure or diabetes.In addition, they increase hospitalization and interruption time, with a real advantage in favor of laparoscopy.
Complications of percutaneous renal biopsy:
The technical improvements of the last 20 years have made it possible to impose the renal biopsy as a safe technique, without major complications in most cases. No complications requiring nephrectomy or surgery have been reported, and only one kidney biopsy-related death has been recorded in the last 20 years, biopsy performed by real-time ultrasound guidance and the automatic gun technique. Nevertheless, the potential for severe complications is still present.
Hemorrhagic complications:
Haemorrhagic complications remain the main cause of renal complication despite the changes in technique. Although their incidence and spectra have not been altered over time, we note a reduction in severe complications and an increase in their management by endovascular gestures. The incidence of haemorrhagic complications is evaluated differently in the literature, depending on the techniques used for their diagnosis. It was 13% in a recent series of 750 renal biopsies performed over 20 years, during which the diagnosis of complications was mainly clinical (hematuria, pain, hypotension, hematocrit reduction) and 34% when ultrasound kidney postbiopsy research was systematic. The persistence of this relatively high incidence over time is explained at least in part by the widening of the contraindications to the realization of renal biopsies in patients at risk, such as patients with a significant drop in filtration rate glomerular (GFR) estimate.
Most complications resolve spontaneously.
However, major complications are reported in 6-7% of all biopsies. These are clinically significant complications requiring therapeutic (radiological, surgical or transfusion of red blood cells) or acute renal failure, obstruction, septicemia or death. More than 90% of major complications and 89% of all complications occur within 24 hours of renal biopsy. No other clinical or radiological element, nor the systematic ultrasound monitoring immediately after the renal biopsy, nor the initial modification of the hematocrit distinguishes patients at risk of severe complications. Thus, the recommended renal postbiopsy period is 24 hours.
• Macroscopic hematuria is the most frequent complication of renal biopsy. In most cases, it merely requires medical supervision and an abundance of drinks. In case of prolonged hematuria or responsible for deglobulation, arteriography allows selective identification and embolization of the artery at the origin of the bleeding. Significant hematuria may be responsible for ureteral or bladder obstruction. Exceptionally, the obstacle on a single kidney can be the cause of acute renal failure.
• Perinatal hematoma occurs in less than 6% of prospectively evaluated renal biopsies. The majority of the hematomas are asymptomatic and small. Their existence justifies the current practice of 24-hour rest in bed, contributing to the decrease in the incidence of clinically significant hematomas. The volume of the hematoma is not a therapeutic decision-making element per se, although most hematomas requiring therapeutic intervention are larger than those of asymptomatic patients.
Immediate ultrasound monitoring of renal postbiopsy has no predictive value on the development of hematomas. No correlation was found between their volume and the different predictors of bleeding.
• Arteriovenous fistula was reported in 10.8% of the kidney biopsies in the series with systematic screening by post-echocardiography, with a significant difference between the native kidneys (4.1%) and the biopsied transplants (16.9%). More than 95% of arteriovenous fistulae close spontaneously within 2 years, although persistence of haematuria has been reported 30 years after renal biopsy. The majority of arteriovenous fistulas are asymptomatic and progress favorably, making screening routine useless. Exceptionally, they can lead to persistent macroscopic hematuria, post-traumatic arterial hypertension (hypertension), or even degradation of renal function, thus imposing selective arterial embolization.
Other complications:
The local pain in the end of the effect of local anesthesia is commonplace and most often does not require treatment.Sometimes the pain may be more severe, requiring even opiates, especially in cases of large perirenal hematoma or renal colic due to ureteral obstruction with blood clots.
A wide variety of other complications have been described: hemothorax, colonic perforation, hepatic or splenic and even pancreatic biopsy, leading to pancreatitis outbreaks. These complications become exceptional with the real-time feedback technique.
Predictive factors of bleeding:
A recent study identified as factors associated with haemorrhagic complications of ultrasound-guided percutaneous renal biopsies:
• sex: the risk is greater for women;
• age: the risk is higher for the two extremes of age (<20 years and> 70 years), whatever the histological diagnosis;
• part-time thromboplastin: the risk of bleeding is increased with each 10% increase in the part-time thromboplastin.
In this study, neither hemoglobin, renal insufficiency, nor bleeding time were predictive of hemorrhagic complications.
Indeed, the use of bleeding time as a screening test before renal biopsy remains highly controversial. Its positive predictive value of bleeding in a non-selected population is low and false negative results would give false security.“The College of American Pathologists” and “The American Society of Clinical Pathologists” do not recognize bleeding time as a routine preoperative test without significant prior bleeding. The empirical treatment with desmopressin (DDAVP) of all the patients before the biopsy brings a better cost-effectiveness.
The last few years have imposed the platelet aggregation test (TAP) as a simple and rapid technique for in vitro evaluation of platelet function. TAP abnormalities are not specific to specific platelet involvement and should be interpreted in conjunction with other tests. Many studies have attempted to compare TAP to bleeding time in the detection of primary haemostasis disorders. A very large and recent pre-surgery study of 5,649 unselected patients showed that TAP had a positive predictive bleeding value of 82% and a negative predictive value of 93%. TAP abnormalities combined with a history of bleeding make it possible to detect the existence of an abnormality of hemostasis in almost all cases. In addition, TAP abnormalities were corrected after DDAVP administration in 90% of cases, leading to a bleeding risk comparable to that of the control group. Even in the absence of specific studies evaluating TAP as a predictor of renal biopsy bleeding, these results justify its use as a screening test before renal biopsy. In addition, TAP can be used in prebiopsy monitoring of patients with hemostasis abnormalities.
Patients with advanced chronic kidney disease (GFR <40 ml / min / 1.73m 2 ) have an increased risk of postoperative bleeding six times greater than those with GFR between 60 and 80 ml / min / 73m 2 , regardless of coagulation status.Treatment with DDAVP, under the control of TAP, may be useful in these patients.
Hypertension is classically recognized as a risk factor for bleeding. This statement is not based on any studies. Gault and Muehrcke argue that the risks are lower if the blood pressure is controlled and the constriction of the vessels is decreased during amyloidosis and vasculitis. The common practice is to accept 140/90 mmHg as the maximum blood pressure threshold before the procedure.
Contraindications of percutaneous renal biopsy:
Most of the contraindications of percutaneous renal biopsies are relative. The only absolute contraindications are the lack of consent and the inability of the patient to cooperate during the procedure. For guardians and minors, the consent of the guardian or parents must be required before the biopsy.
The risk of bleeding should be minimized before and during the 2 weeks following renal biopsy, requiring suspension of antiplatelet agents, nonsteroidal antiinflammatory drugs and anticoagulants. A higher risk of bleeding persists up to 6 weeks after the biopsy, as for any healing process that involves the fall of pressure sores. The inability to rule out the factors favoring bleeding intervenes in the choice of the renal biopsy technique. The existence of persistent thrombocytopenia, the use of antiaggregants in patients with active coronary stents or anticoagulants in patients with extensive venous thrombosis or pulmonary embolism justify the choice of the transvenous route.
The single kidney is a contra-indication “relative” to the percutaneous biopsy. The endovenous pathway can be proposed as an alternative biopsy of a single right kidney. Both techniques have a low risk of complications requiring nephrectomy. This risk is considerably lower than the risk of death by general anesthesia, which does not justify the choice of the surgical route.
The “horseshoe” kidney was considered as a contraindication to the renal biopsy without being able to determine whether this was due to positional abnormalities or the unique character of the kidney.
The journals on the subject date from before the use of ultrasound guidance. A good visualization of the vascularization of this kidney is to be proposed.
Indications of renal biopsy:
Diagnostic intake of renal biopsies is indisputable for four indications: nephrotic syndrome, systemic diseases with renal involvement, acute renal failure (ARI) and renal graft dysfunction. Renal biopsy may also be useful from a diagnostic and therapeutic point of view in patients with non-nephrotic flow proteinuria, hematuria or chronic kidney disease.
Nephrotic syndrome:
There are two exceptions to the renal biopsy rule for nephrotic syndrome:
• children between 1 and the age of puberty, in whom we can first retain the diagnosis of glomerulopathy with “minimal glomerular lesions” (LGM), in the absence of atypical elements, such as the decrease in C3, l the existence of hematuria and / or renal insufficiency.
These patients receive a protocol of corticosteroid therapy, the biopsy being only carried out in the corticoresistant forms.
In children less than 1 year of age in whom LGM is rare, biopsy is indicated from the outset to identify other etiologies of nephrotic syndrome for which the diagnosis is histological and genetic;
• adults with diabetes, with a long history of diabetes, with a progressive increase in the proteinuria flow, up to the nephrotic flow. The presence of macroalbuminuria or microalbuminuria in patients with a diagnosis of diabetic nephropathy in the absence of renal biopsy in patients with diabetes mellitus (type I) at least 10 years old or with diabetic retinopathy Guidelines KDOQI. Renal biopsy is indicated in cases of absence of diabetic retinopathy, accelerated degradation of GFR, sudden increase in proteinuria, presence of active urinary sediment or signs of other systemic diseases when other etiologies are to be considered .
Systemic diseases associated with proteinuria or renal insufficiency:
Multiple systemic diseases such as amyloidosis, myeloma, sarcoidosis or drug-related complications can be diagnosed by renal biopsy. For vasculitis and systemic lupus erythematosus (SLE), renal biopsy allows classification: the Chapel Hill classification for systemic vasculitis and the classification of the International Society of Nephrology and the Renal Pathology Society, glomerular involvement of patients with SLE. The aim of these classifications is to define more precisely the renal impairment in order to better orient the therapeutic protocols and to refine their prognosis. Renal biopsies may indicate the degree of disease activity, and may be used to evaluate the effectiveness of the treatment.
Acute renal failure:
The diagnosis of patients with ARI is mainly focused on acute tubular necrosis, particularly in a clinical context suggestive of renal hypoperfusion. However, renal biopsy is indicated, and as soon as possible, for any patient with ARI of unknown aetiology in order to allow a diagnosis of certainty and to initiate an appropriate treatment. The renal biopsy during the ARI is indicated in case of:
• absence of recognized cause;
• urinary albuminuria / creatinine ratio> 30 mg / g in favor of glomerular disease;
• significant hematuria;
• Recent HTA;
• Prolonged oliguria.
The presence of haematuria and pathological albuminuria accompanying the IRA leads to glomerular involvement, such as vasculitis. In the case of vasculitis, which are therapeutic emergencies, the renal biopsy regains its value in order to confirm or confirm the diagnosis and to establish the severity of inflammatory reactions and the extent of fibrosis. When the extrarenal signs are not very evocative, the renal biopsy can reveal specific lesions of necrotizing angiitis. They may be encountered in systemic diseases such as SLE, rheumatoid arthritis and cryoglobulinemias; they can occur in the course of bacterial or viral infection, after taking a toxic or in the course of certain malignant diseases.However, most necrotizing angels with renal involvement are considered primitive: Wegener’s granulomatosis, microscopic polyangiitis, Churg syndrome and Strauss syndrome, closely associated with anticytoplasmic antibodies to polymorphonuclear cells (ANCA). The study in immunofluorescence is oriented towards an etiological research that distinguishes a rheumatoid purpura, an endocarditis with post-infectious glomerulonephritis, and especially a Goodpasture syndrome. However, it is conceivable to postpone the renal biopsy if the initiation of treatment has been imposed by the extrarenal manifestations of the vasculitis. Nevertheless, the diagnosis must be a diagnosis of certainty because it is difficult to decide a heavy immunosuppressive treatment without histological evidence.
Renal biopsy may also be useful at a distance from the initiation of treatment to assess the therapeutic response and thus to judge the desirability of continuing immunosuppressive therapy.
Proteinuria of non-nephrotic order:
Proteinuria is a marker but also a prognostic factor for renal disease. Several studies have shown an acceleration of the degradation of renal function in relation to the flow of proteinuria. The loss of GFR is evaluated at 0.4 ml / min / month in patients with proteinuria between 1.5 and 3 g / 24 hours. Thus, it is important to establish the diagnosis of nephropathies in patients with proteinuria> 1.5 g / 24 hours, although sometimes the therapeutic implications remain modest. The indication of renal biopsy is reinforced by the clinical context, as for example during rheumatoid arthritis with proteinuria.
If AA amyloidosis is detected, anti-inflammatory therapy should be intensified, whereas extramembrane glomerulopathy (GEM) should stop gold salts or penicillamine.
Isolated hematuria:
The indication of renal biopsy in this situation remains controversial. After removal of the urological causes of haematuria, the most probable diagnoses remain nephropathy with fine basement membranes and immunoglobulin (Ig) A nephropathy.
Chronic unexplained renal insufficiency:
Renal biopsy can provide important information, especially if kidney failure accelerates. In this case, it may reveal lesions justifying specific treatment (extracapillary proliferation, cholesterol emboli, interstitial nephropathy).
It may also be important that an unknown diagnosis is made even at an advanced stage of renal insufficiency for possible renal transplantation, which will influence post-graft surveillance and management: primary or digestive oxalosis, amyloidosis, etc. Nevertheless, in the presence of chronic renal failure with small kidneys, renal biopsy is dangerous.
Renal graft dysfunction:
Biopsy is particularly useful in early graft dysfunction or delayed function recovery. It allows to confirm the diagnosis of rejection and also to specify its pathological mechanism (acute rejection mediated by antibody or acute rejection cellular). This is an essential step for the rapid introduction of an effective treatment, such as intravenous immunoglobulin (IVIG), plasmapheresis and anti-CD20, for the treatment of acute antibody-mediated rejection. Late biopsies also provide essential information to differentiate acute cellular rejection or antibody-mediated rejection, from nephrotoxicity to calcineurin inhibitors or viral nephropathy (BK virus nephropathy), or lymphoproliferation, or d a graft injury by the initial kidney disease, etc. The simplicity of the technical gesture combined with the wealth of diagnostic and prognostic information makes the biopsy indispensable in the monitoring of kidney transplants. Teams have shown a short- and long-term benefit of routine biopsies of the renal graft.