SLOW MOTION CIRCULATING MELTING MALFORMATIONS:
SLOW MOTION CIRCULATING MELTING MALFORMATIONS:
Those of the capillary compartment (telangiectasia) or the venous compartment (cavernoma) begin to be discovered today by the MRI. Rare, they are sometimes responsible for hematomyelia or spinal cord compression. The venous pockets give a heterogeneous signal in hypo- and hypersignal due to stagnant blood, and sometimes in case of bleeding the presence of a peripheral border of hemosiderin hyposignal.
Interventional radiology currently has no place in their treatment.
FAST CIRCULATION MELULATIVE MALFORMATIONS:
Early diagnosis is important because the clinical course depends on an effective treatment, performed early after the appearance of the symptomatology. Three groups of vascular malformations dominate: medullary arteriovenous malformations (AVM), peri-arterial arteriovenous fistulas (AVF) and spinal meningeal FAF. MRI is becoming increasingly important in the early diagnosis.
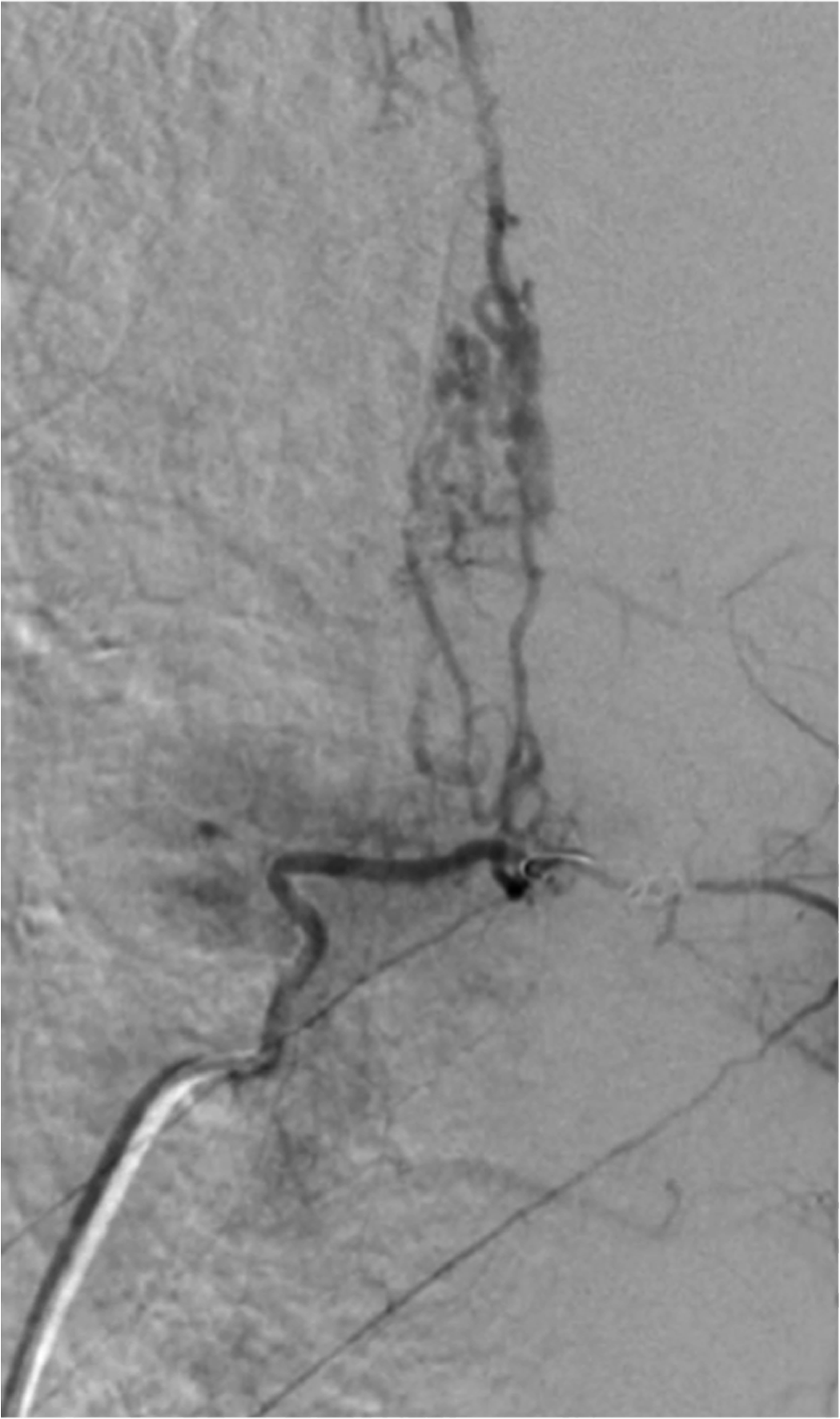
The arteriography introduced by Djindjian (1962), alone allows to specify the angioarchitecture, opening the door to endovascular treatment by spinal embolization.
Medullary arteriography remains the indispensable diagnostic examination. Under local anesthesia and light sedation, it determines the anterior and posterior spinal feeder arteries by selective catheterization of the cervical, intercostal, lumbar and sacral arteries, knowing that an initial global study by aortic opacification (or bilateral retrograde humeral for the cervical region) can simplify exploration.
Currently, exploration is digitized. Its resolution must be sufficient to detect a dorsal anterior spinal artery (ASA) of 300 μm caliber. The anterior spinal vein is the main vein, but a posterior spinal vein can become opacified by the anastomotic network. The medulloradicular veins are well individualized. The normal perimedullary veins of the upper and middle dorsal regions by their anatomical configuration are not visualized. A MAV has dilated arteries and spinal veins.
Intramedullary arteriovenous malformations:
Definition:
They are partly or wholly in the depth of the marrow. Most often at high flow rates, they are multi-pediculated, almost always fed by ASA and one or more posterior spinal arteries. They are drained by dilated veins of the spinal cord towards one or more radiculoepidural efferent veins, close to the seat of the MAV. These are arteriovenous malformations because between the afferent arteries and the efferent veins, there are multiple arteriovenous shunts constituting the “nidus”.
Haemorrhage and ischemia by hemodotournement are the main lesional factors.
Clinic:
They are revealed in the child or the young subject with a slight male predominance. The evolution is made of partially regressive thrusts: spinal meningeal haemorrhage or hematomyelia. The radiculomedullary deficit syndromes (paresthetic and painful sensory disorders, motor disorders, sphincter disorders), progressive installation are possible and misleading (multiple sclerosis).
These rare malformations are of serious prognosis: 20% mortality in the acute phase. When the patient survives the bleeding episode, the neurological sequelae responsible for decubitus complications are life-threatening.
Additional tests :
Study of the cerebrospinal fluid (CSF):
It gives variable results: normal CSF, haemorrhagic or with albuminocytological dissociation.
Standard radiographs of the spine:
They rarely show enlargement of the spinal canal suggestive of an intraductal expansive process, erosion of the posterior border of the vertebral bodies or of a pedicle.
Myelography:
Associating standard snapshots and tomographies (face, profile), for a long time the first examination practiced in the exploration of myelopathies, it provides direct images of the perimedullary vessels, but indirect images of the marrow;it can highlight an image suggestive of large marrow associated with abnormal vascular images. Excluding pathology, the anterior spinal vein and the veins of the ponytail are never visible, the posterior spinal vein is visible in the lower and lumbar dorsal regions, but never above T4 and over 8 storeys. The amplitude of the sinuosities is lower than the diameter of the marrow.
The medulloradicular veins of the terminal cone are often seen.
Computed tomography examination of the marrow:
We can not go further in the diagnosis.
Magnetic resonance imaging :
Noninvasive, it gives a direct image of the marrow and perimedullary spaces. It does not visualize the normal medullary vascularization because its power of resolution is still insufficient. It provides the diagnosis, specifies the intra- or extramedullary location of vascular dilatations and detects the associated complications (thrombosis, intramedullary hematoma, hemorrhage, medullary pain). The angio-MRI has an interesting future to specify.
Selective arteriography of the arteries:
Giving the ASA and ASP (posterior spinal artery), it determines volume, flow, exact topography, height and transverse extension, spinal pedicels, venous drainage and the possible presence of arterial vascular ectasia and / or or venous.
The superficial AVMs may be of superficial intramedullary localization (cordial for example) or with a pial extension and more rarely completely pial. The superficial components of a marrow AVM are identified through angiography and MRI.At angiography, the pure pial components are irrigated by ASPs also called posterior radiculomedullary (RMP) or radiculopial, because the normal territory of ASP is 80% radiculopial and 20% intramedullary superficial. The same arteries will irrigate a superficial intramedullary AVM of posterior or lateral location. The ASA sometimes irrigates a pial compartment of an MAV by circumferential piales, but this irrigation will always be coupled with the participation of the PMRs. Another way of distinguishing the pial compartment from a malformation is the demonstration of a malformative extension towards the nerve root. In summary: an AVM irrigated solely by ASA is deep and anterior and an AVM with an RMP participation has an associated surface component.
Two types of venous drainage are distinguished: local fast-flowing venous drainage with dilated veins and extradural outflows close to the malformation is the classical drainage of an AVM; Extensive venous drainage at a slower rate in small veins, and out of the intradural spinal area, away from the MAV, through the veins of the ponytail and / or through an ascending venous system to the veins of the fossa posterior is rarely encountered in AVMs. It is similar to venous drainage of perimedullary or meningeal FAV. The symptomatology is no longer brutal, in fits and starts, rich in meningeal hemorrhages with sensitivomotor deficits, but with progressive ascending myelopathy.
Treatment:
The embolization proposed since 1970 was a considerable contribution by initially allowing the preoperative drying of the malformation. Since 1978, the clinical and angiographic results of curative embolization have been such that embolization has become the first, and often the only, treatment, even in pauci- or asymptomatic patients (prevention of a subsequent accident). . Thrombosis of AVM is investigated using calibrated microparticles that are driven to malformation by the predominant flow. These particles must stop in the shunts while respecting the carrier artery and the normal medullary arteries. The embolization by an ASP is considered less dangerous, contrary to the embolization by an ASA where one must respect certain absolute rules: control of the existence of sulcocommissurales wider than the ASA downstream of the MAV allowing to take the embolization particles preferentially to the MAV. The result of this particle embolization is regularly good. The earlier the embolization, the better the clinical recovery. However, reversal is usual, requiring systematic angiographic testing every year or every 2 years. A new embolization is then performed.At this price, the long-term evolution is favorable. Even partial particle embolization can definitely change the natural history of AVM with the disappearance of hemorrhagic risk and acute accidents. Polymerizing substances (N-butylcyanoacrylate), a biological glue, are used only if superselective catheterization of AVM is possible and only if respect for normal spinal arteries is safe. His risk is greater, but the occlusion obtained is final. It is difficult to complete and a residue must also be monitored. In the current state of the art, most intramedullary AVMs treated with polymerizing substances are of cervical localization, because the arteries have a short path and simpler to catheterize selectively making it easier to come into contact with the malformation. Medullary monitoring by electrophysiological methods (evoked potentials and medullary somatosensory) used by some authors, has no predictive value for the marrow risk of embolization. In the end, we can only rely on the angioarchitectural study, the understanding of the hemodynamics of the MAV and the identification of substitutions to the adjacent normal marrow. Posterior median commissurotomy surgery only has a place in second intention (contraindication to embolization, exhaustion of embolization possibilities) if the nidus is compact, median, away from ASA with arteries sulcocommissurales long, and if the dominant venous drainage is not posterior, which hinders the approach.
These intramedullary AVMs are isolated but in 30% of cases they are part of metastatic metastatic angiomatosis, of which Cobb syndrome is one of the aspects.
In summary, intramedullary AVMs are usually noisy in young patients. The prognosis is dominated by haemorrhagic events and, without treatment, the functional and vital prognosis is dark.
In this treatment embolization takes a dominant place, even if, usually made with particles carried by the flow, it exposes to revascularization and therefore requires arteriographic surveillance (with or without reembolization) annual.The use of a polymerizing material is more particularly indicated in surface malformations (with posterior spinal input), or in the case of possible intranidus catheterization. The intervention, finally, should be considered when the topography is well defined and the MAV well controlled by a prior embolization.
Intradural perimedullary arteriovenous fistulas:
Definition:
They correspond to a full-channel direct (not intramedullary) and variable-caliber (3 types, 3 haemodynamic) direct shunt between a spinal artery and a perimedullary vein, often located in the area of the spinal cone. and the ponytail.The mechanism of the medullary pain is a deep circulatory slowdown of the spinal cord related to hemodontournement and venous hyperpressure (arterialization) and venous stasis. A mass syndrome is added in the bulky, or giant forms of the child.
Clinic:
They are generally revealed in young adults (20 to 40 years) by meningeal suffering. Interrogation often finds the notion of “meningism” or an acute spinal neurological accident in childhood. The giant forms of the child are responsible for a progressive or sometimes brutal deficit, evolving to paraplegia very quickly (in a few months to 2 years).
Imaging:
Myelography typically visualizes vascular abnormalities, confirms the full-channel shunt on the surface of the marrow which is of normal caliber, thus affirming the perimedullary character. MRI shows the perimedullary vessels and medullary pain possibly associated and correlated with the existence of myelopathy. Angiography confirms the full-channel shunt and classifies fistulas into three groups according to the size of the shunt and the morphology of the arteries and veins.
Type I arteriovenous fistulas:
These are very low rate fistulas between an almost normal ASA and a small drainage vein. The drainage is extensive, with no epidural effeciency locally identifiable. They sit properly in the cone region. They are difficult to diagnose angiographically, and many of them must probably be misunderstood.
Type II arteriovenous fistulas:
These are fistulas with greater flow, uni- or multipédiculées (ASA, RMP). Venous drainage is here dilated simple or complex and extensive away from the seat of the fistula (which justifies the symptomatology of medullary suffering by venous stasis), fistulas type II have the same clinical features because of their extensive venous drainage and away from the shunts. The venous side of the arteriovenous shunt can usually be seen as ampullary ectasia. They also predominate in the cone region and the ponytail.
Type III arteriovenous fistulas:
They are giant fistula with high flow fed by multiple pedicles, one of which, very dilated, is dominant. They are located on all floors. Their drainage is ensured by often monstrous ectatic veins which are here with locoregional drainage.This is a very distinctive difference between types I and II and type III.
After studying 34 FAV cases, certain trends are observed: clinically there is a male predominance, especially for late-stage progressive disclosure FAV I (4th decade). FAV II and III may be haemorrhagic and frequently have radiculopathies.
Treatment:
The gravity of evolution encourages us to treat these malformations even if they are fortuitous discovery.
Early treatment, ie closing the fistula and occlusion of the foot of the drainage vein, respecting the normal arteries and spinal veins, is the determining factor in clinical recovery. Surgery is reserved for type I fistulas, ie small fistulas, difficult to access to embolization because arteries are very thin and remote embolization presents major risks. In type II shunts are rarely affected by particle embolization because they can pass beyond the shunt and effective endovascular treatment should be directed to superselective catheterization and injection of curing substance.
When it is not possible, surgery should be offered. Surgery is delicate in case of large posterior vascular dilatation or shunt on the anterior side of the cord. In type III, giant, the treatment is reasonably endovascular, using, depending on the case, a balloon, coils and / or polymerizing material.
In summary, it is a direct, full-channel AVF on the surface of the spinal cord, typically revealed in a young adult by slowly progressive myelopathy (10 years).
Morphologically, venous drainage is extensive and the flow is slow or moderate. Only the final closure of the shunt can ensure healing.
A particular form consists of the giant forms, also observed in children, with a more noisy, painful and haemorrhagic manifestation. The drainage is here local. Treatment can only be endovascular.
Arteriovenous spinal meningeal fistulas with peripedullary venous drainage:
Definition:
They are characterized by a microshunt located in the thickness of the dura mater, between an artery with radiculomeningeal destiny and a single-rule medulloradicular vein contaminating extensively the medullary venous network without association of local epidural drainage. Venous drainage is “extensive” in small veins, at a very slow rate, almost stagnant to veins of the ponytail (one-third) or ascending to the posterior fossa, which are the only extradural venous outflow pathways. It is accompanied by a medullary venous hyperpressure hindering the normal venous drainage of the marrow and responsible for the clinical picture of the “subacute necrotic myelitis” described by Foix and Alajouanine in 1926. The origin of the fistula, is not yet well determined, but it seems to be acquired.
Clinic:
They represent 50 to 80% of medullary vascular malformations following series. They are usually found in men (5 men / 1 woman) in their fifties (40-80 years), in the form of progressive myelopathy worsening until paraplegia, in about 2 years. The onset is most often insidious by asymmetric neurological involvement of the lower limbs (central radiculalgia or peripheral L4-L5 or S1, paresthesia type burns or tingling), by misleading urinary disorders, the attack can be only motor to type of medullary claudication. The evolution in a few months is towards an associated sensitivomotor and genitosphincteric lesion. At the time of diagnosis, myelopathy is manifested by different neurological charts, in order of decreasing frequency: cone or horse tail syndrome, and isolated spasmodic paraparesis. The sensory level is never superior to D10, and the medullary suffering remains backless, regardless of the seat of the fistula that can be dorsal high or low, lumbar or sacral. Numerous diagnostic errors (alcoholic or diabetic polyradiculoneuropathy, narrow lumbar canal, late multiple sclerosis) delay the formal indication of arteriography. This type of fistula never bleeds.
Additional tests:
Study of cerebrospinal fluid:
It may reveal a moderate and isolated increase in proteinase.
Dorsolumbar myelography:
It has fine tomographic sections and shows, in the typical form, a dilatation of the retromedullary veins, tortuous, extended on the thoracolumbar or even cervical cord.
Magnetic resonance imaging :
This is the exam of choice. It can highlight two elements: venous dilatations, but above all there is a high signal, in T 2weighted sequence witnessing the medullary pain of the cone which is often increased in size. Even in the absence of perimedullary vascular images, this hypersignal of the cone should indicate the medullary angiography and not the bone marrow biopsy or the surgical procedure by confusion with a tumor of the cone.
Normal MRI (without visible perimedullary vessels) does not eliminate the diagnosis of dural FAV. Do not hesitate to ask for a marrow angiography in front of a progressive myelopathy chart in a subject over 50, especially if there is a high signal of the cone (in T 2 ) because it is one of the rare curable “medical” myelopathies with good prognosis when diagnosis and treatment are early.
Angiography:
It aims at two elements: the fistula and the absence of venous return of the ASA of the cone (artery of Adamkiewicz).After injection into anterior radiculomedullary artery, the normal venous return of this artery must be visualized within 12 to 15 seconds after the injection, (ie the visualization of the medullary vein (s) (s) ( s) and epidural effect). In this condition, this drainage is not identified, which is a characteristic sign of venous hypertension. The fistula, low flow can sit on all floors (from T3 to S3). This shunt is microscopic; it is in the thickness of the dura mater and drains by a single vein of root type anterior and / or posterior, ascending and / or descending. The drainage is slow with only visible exits either root veins of the ponytail (one-third) or cervical ascending veins and / or posterior fossa.
Treatment:
Treatment, if it is early, involves not only stabilization, but functional motor recovery, sensory and especially sphincteric and genital recoveries are delayed and inconstant. The size of the shunt requires the use of a fluid embolization material (cyanoacrylate) whose polymerization time is modulated by the addition of Lipiodol ® . The treatment consists in very selectively occluding with this “glue” the shunt and especially the foot of the drainage vein thus avoiding a repealization by a meningeal anastomotic network. The venous return of ASA reappears in the following months. If the ASA is born at the same level as the FAV or if there is uncertainty as to the obliteration of the foot of the vein or the technical risk of embolization, surgical clipping is necessary. Lack of clinical improvement after a few weeks or a worsening should be done to indicate a test anticoagulant treatment.
The hypersignal of the cone may disappear between 2 months and 3 months after treatment.
Other locations:
Intracranial dural arteriovenous fistulas with medullary venous drainage:
They correspond to a new entity, draining into the perimedullary veins. They correspond to type V of the classification of intracranial dural fistulas. The diagnosis is difficult and late because the signs of clinical and radiological calls are medullary while the fistula is visualized only by carotid or vertebral arteriography. The first observation is that of an ascending myelopathy beginning with a deficit of the lower limbs and sphincter disorders and complementing by an attack of the upper limbs, then cranial nerves. But many other paintings exist: resolutive paraparesis, tetraparesis from the outset, radiculopathies or radiculalgies. The evolution is done in fits and starts. Myelography and MRI show extensive dilated small perimedullary vessels with or without extensive hypersignal. Venous drainage of Adamkiewicz’s artery is absent recalling dural dural FAVs. Only the arteriography of the supraaoretic trunks will authenticate the AVF, which may be located on a lateral sinus, the region of the tent or the occipital foramen. Embolization is the treatment of choice.
We also bring this type of pathology closer to other medullary pain of venous origin: in relation with epidural or extraspinal MVA; abnormalities of the basement system with probable epidural collateral circulation; spinal thrombophlebitis.
Epidural and foraminal arteriovenous malformations:
They are rare. They can have a radicular or medullary impact, especially if their venous drainage borrows perimedullary veins. The symptoms are varied: isolated radiculalgia, progressive myelopathy simulating dural spinal FAV, subarachnoid hemorrhage, medullary compression table. All these tables correspond to various angioarchitectures which can not be detailed here.
SPINAL VASCULAR MALFORMATIONS:
Spinal angiomas:
Clinic and Imaging:
Vertebral angiomas of the adult that should be called venous malformation or capillarveinous vertebral correspond to a venous or capillary abnormality, intraosseous, solitary rule, sometimes multiple (vertebral and parts of bone parts). It is an extremely common malformation (10% of subjects) but only a small percentage is symptomatic.
The majority of “images of vertebral angioma” accidentally discovered on radiographs, correspond to a simple quiescent, venous and fatty dysplastic form, localized, intraosseous, non-progressive, in dorsal low or lumbar ruler, painful pure or asymptomatic. This quiescent form has a very characteristic radiological appearance with a grid and striated parcel of the vertebral body. The CT scan shows a greasy and / or veinous honeycomb appearance respecting the corticals without any extension, nor reaching the posterior arch. The MRI of the asymptomatic hemangioma presents itself with a hypersignal on the images T 1 decreasing on the images T 2 testifying to the greasy content.
In contrast to this benign vertebral angioma, which is quiescent, it is an evolutive, expansive angioma revealed by medulloradicular compression, often rapidly progressive at the dorsal or thoracolumbar level. Standard X-ray reveals total body involvement, blown and demineralised cortical appearance, paravertebral (spindle) extension, posterior wall involvement, or posterior arch extension. Myelography shows epidural extension with narrowing or stopping of the contrast column. The CT scan is the reference examination revealing an irregular polycystic pattern, complete involvement of the vertebral body and posterior arch, epidural extension and / or paravertebral soft tissue. In MRI, aggressive vertebral angiomas present a hypointense T 1 and a variable T 2 signal depending on the vascularization.The epidural extension in the soft parts is well visualized. These lesions are enhanced after gadolinium injection.Arteriography shows capillary and venous hypervascularization without early venous drainage. (For the record, vertebral angioma of a Cobb metameric syndrome is a MAV.)
Between the benign angioma with non-progressive fatty or venous composition, and the invasive angioma of evolutive “capillary” structure is the capillarovenous or transition angioma which, in standard radiology, resembles benign angioma and which, at over the years, may progress to invasive angioma.
This is why radiological checks are necessary, even when these angiomas are asymptomatic and have a benign appearance (possibly puncture biopsy).
Treatment:
Therapeutic indications are of interest only to symptomatic vertebral “angiomas” responsible for localized pain, ring-root radiculitis, or compressive medullary syndrome. The installation of a deficit is rapidly progressive, sometimes over several weeks.
Painful vertebral “angiomas” and progressive vertebral angiomas are effectively treated by percutaneous embolization, or vertebroplasty, which has supplanted endovascular embolization. This technique consolidates percutaneously the vertebral segment achieved using acrylic cement.
This intervention carried out under neuroleptanalgesia and under scopic and / or CT guidance consists of bilateral puncture posterolaterally or transpedicularly, using a 10-gauge trocar, the vertebral body. The time of solidification of the cement is adapted to the circulatory speed of the malformation. Complementary injections, with a smaller trocar, can be performed at the posterior arch. In case of spinal compression, a surgical laminectomy is usually necessary.This treatment is greatly facilitated by preoperative embolization which reduces bleeding. Other treatments such as radiotherapy, surgical corporectomy, are possible; only rigorous evaluation leads to consensus on the decision tree.
Paravertebral arteriovenous malformations:
Like the previous intraductals, they have a varied angioarchitecture.
Attentive or adjacent to the rachis, they are rare, isolated or associated with a medullary angioma: posterior and paraepinous, lateral parietal (intercostal space for example), costovertinal angle and hole of conjugation with or without epidural extension. They are often giant and very high flow, being able to be responsible in the young adult of a progressive medullary deficit by hemodétournement, by epidural or medullary venous hyperpressure, by spinal cord compression; elsewhere, they can induce heart failure or paravertebral subcutaneous mass. The scanner and the MRI specify the extension and the repercussion on the neighborhood tissues. Interventional radiology, an almost exclusive treatment, has transformed the prognosis of these malformations by attempting to eradicate them permanently.
COMPLEX FORMS:
Metameric:
They associate a spinal arteriovenous malformation, and especially intramedullary, to a cutaneous angiomatosis (plane angioma). Cobb syndrome is an example of this angiomatosis that associates a metameric angioma and a medullary angioma. The spinal localization MAV can be an intramedullary or perimedullary, dural, or AVM of the vertebral body. A superficial capillary malformation (plane angioma) is the skin marker in 20% of cases. The cervical and lumbosacral metameric space may extend to corresponding members, in a unilateral manner. The embolization treatment should be on the symptomatic segment. Medullary evolution is sometimes related to that of extravertebral angiomatosis: spinal haemorrhages have been observed during the progressive onset of arteriovenous malformation of an apparently isolated limb.
Disseminated:
Rendu-Osler-Weber syndrome corresponds to an autosomal dominant disseminated telangiectatic capillary malformation. The nasal and digestive mucosa are the site of telangiectasia that cause epistaxis and gastric bleeding.They can be associated with arteriovenous malformations of various locations frequently pulmonary but also intracranial or intraspinal.
Modern imaging, advances in medullary arteriography, and the grouping of cases in specialized teams have led to a better understanding of the angioarchitecture, the exact location, the mechanism of medullary suffering, advancing the treatment of malformations. vascular, vertebromedullary
Vascular malformations, vertebromedullary are one of the many areas where interventional radiology has gradually become the treatment to consider in first intention. The importance of early diagnosis must be emphasized. If the indication of an arteriogram that will make the diagnosis of vascular malformation during an acute spinal cord neurology is obvious, it is quite different in case of progressive dorsolumbar myelopathy.
Many of them remain unknown and therefore without treatment. We will end by giving an essential rule of conduct: any myelopathy, whatever the type and location, any unexplained and disabling radiculopathy, especially if it is mobile, must have an angiographic assessment, including cervicocephalic. Angiography today is at almost zero risk, and the greatest risk for the patient would be to miss a treatable vascular malformation.